sodium electron configuration Newton Desk
sodium electron configuration Newton Desk
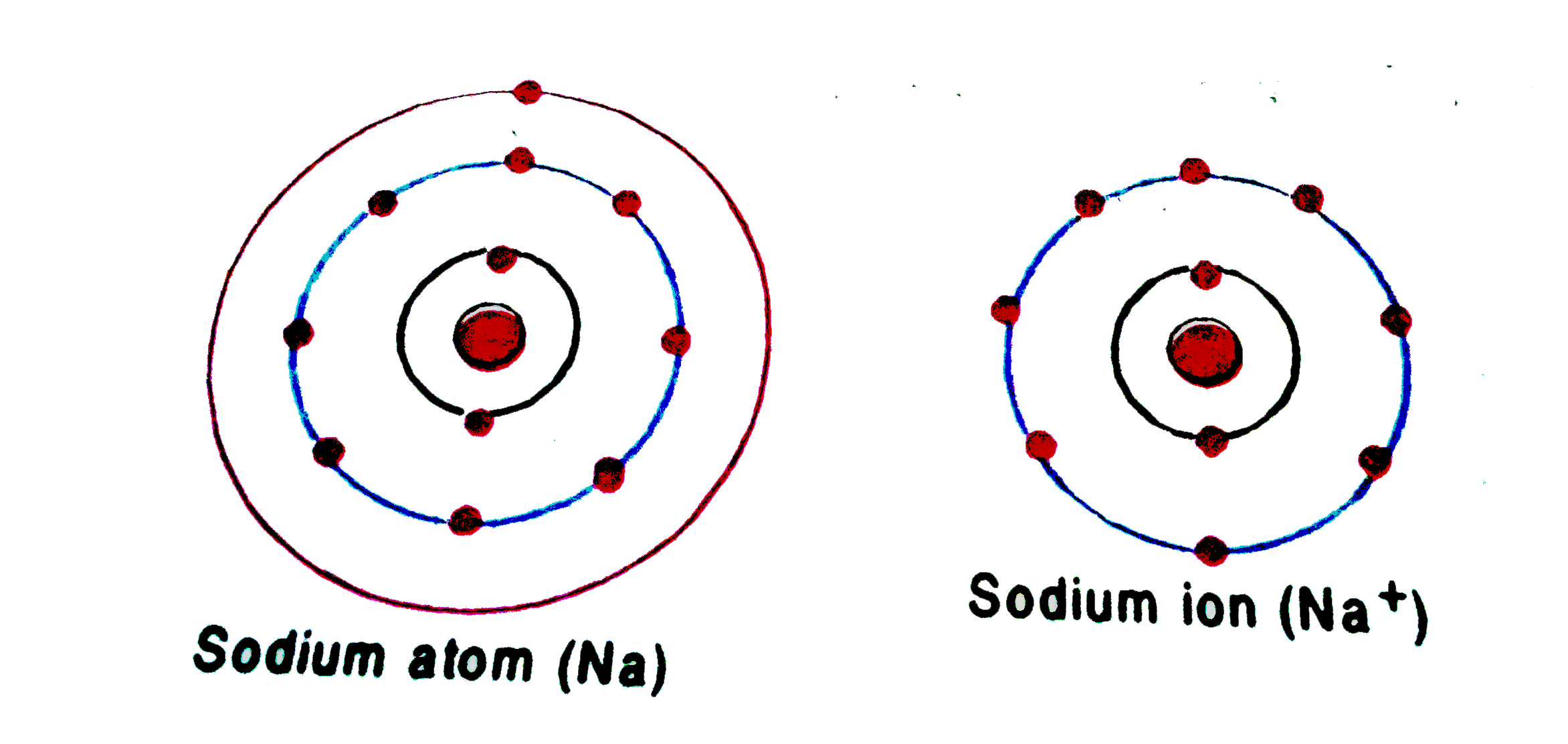
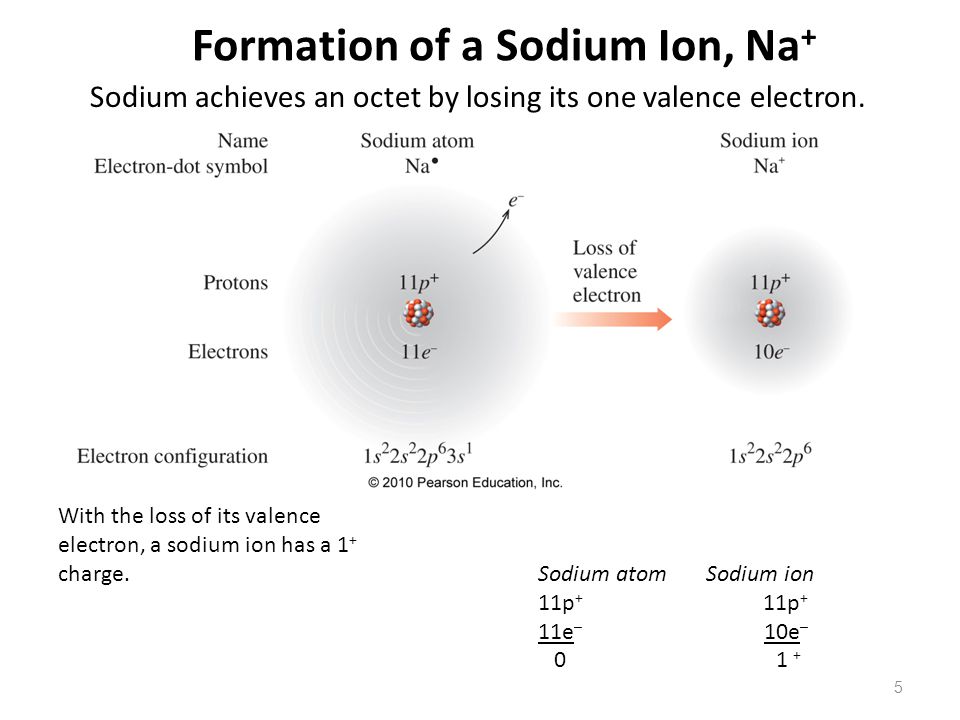
The atom that loses an electron becomes a positive ion. The atom that gains an electron becomes a negative ion. A positive and negative ion attract each other and form an ionic bond. Summary Students will look at animations and make drawings of the ionic bonding of sodium chloride (NaCl).
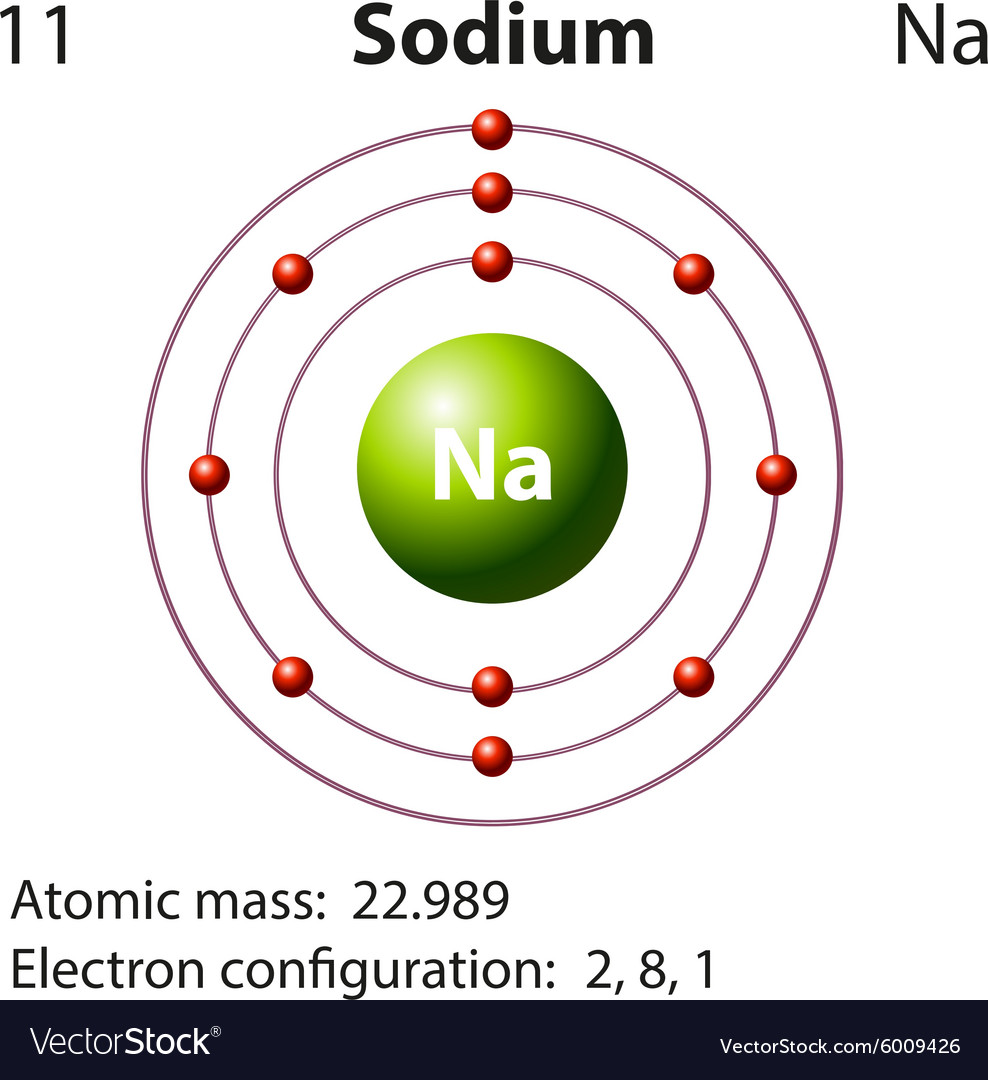
Diagram representation of the element sodium Vector Image
In this video we will write the electron configuration for Na+, the Sodium ion. We'll also look at why Sodium forms a 1+ ion and how the electron configurati.

Sodium Atom Science Notes and Projects
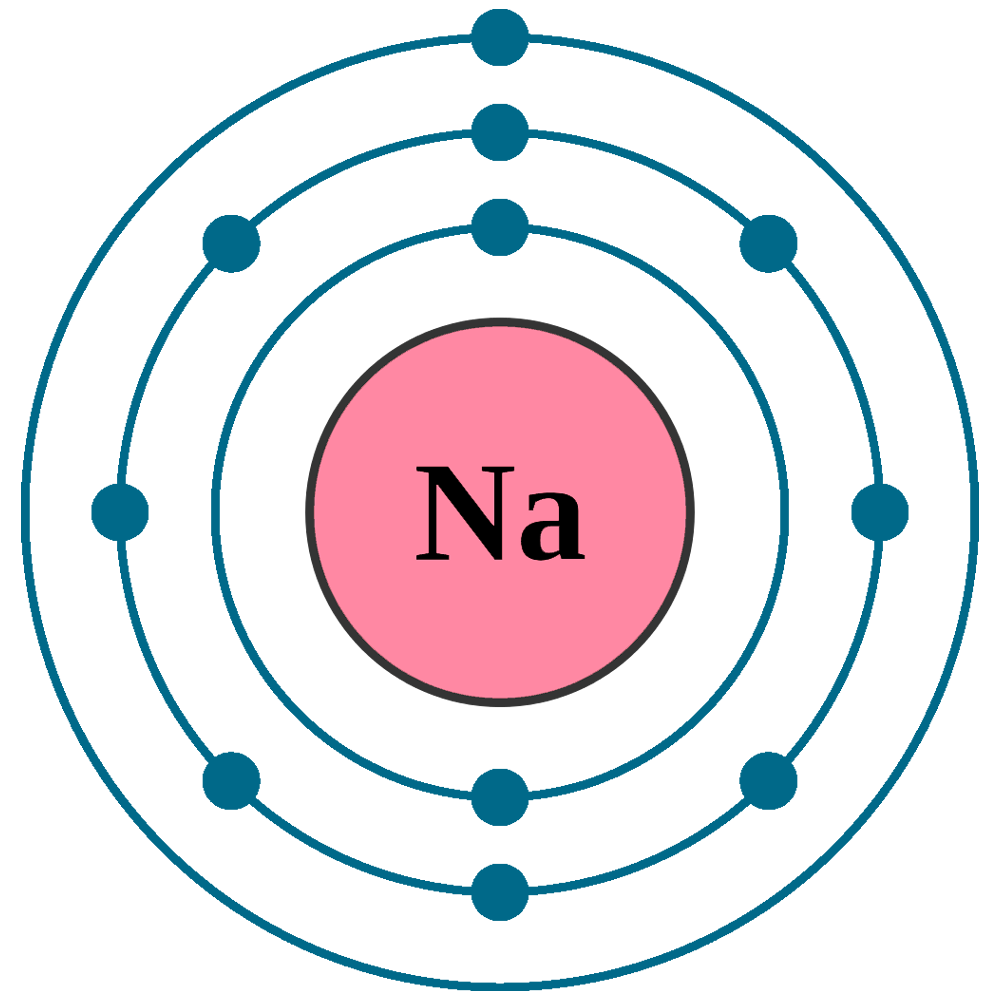
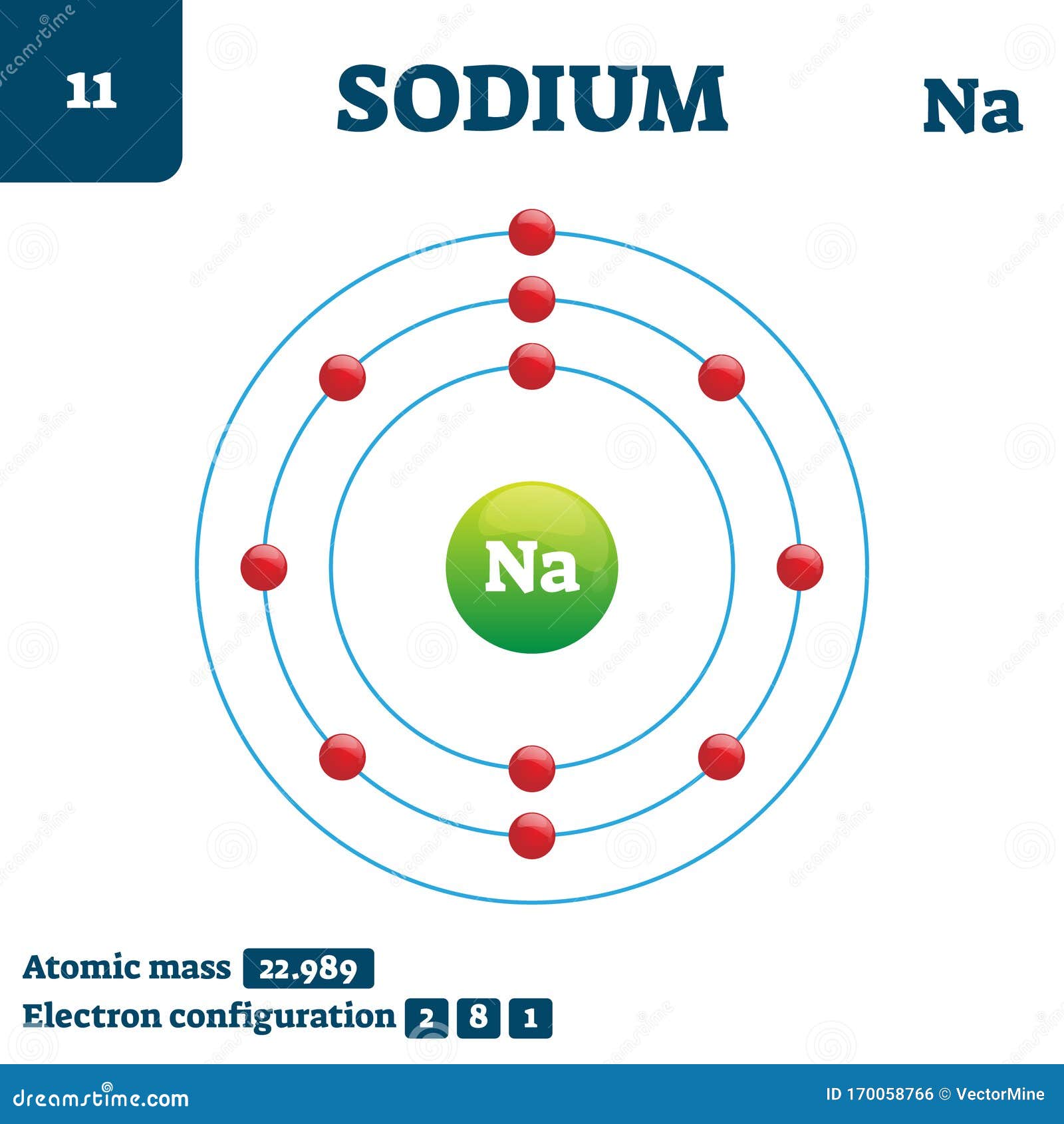
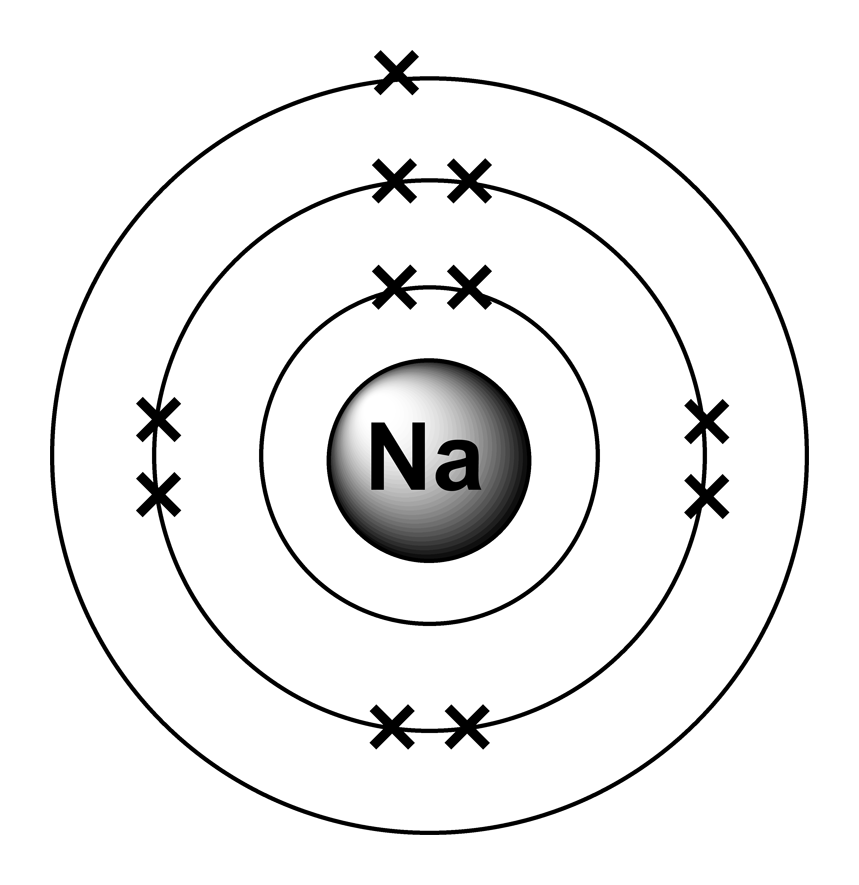
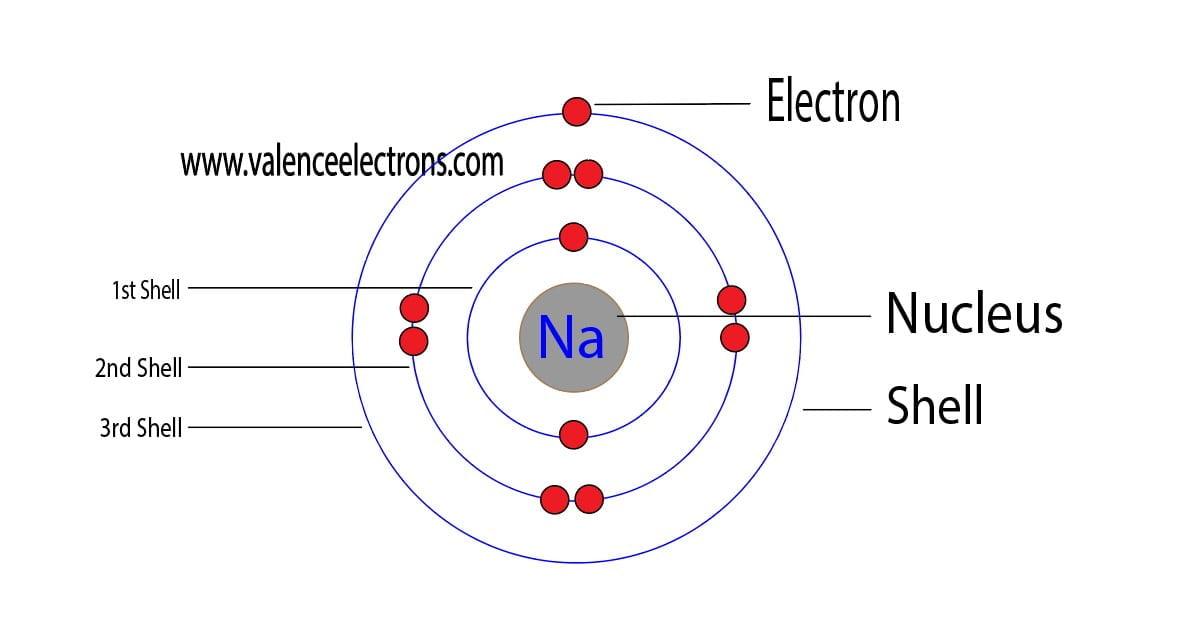
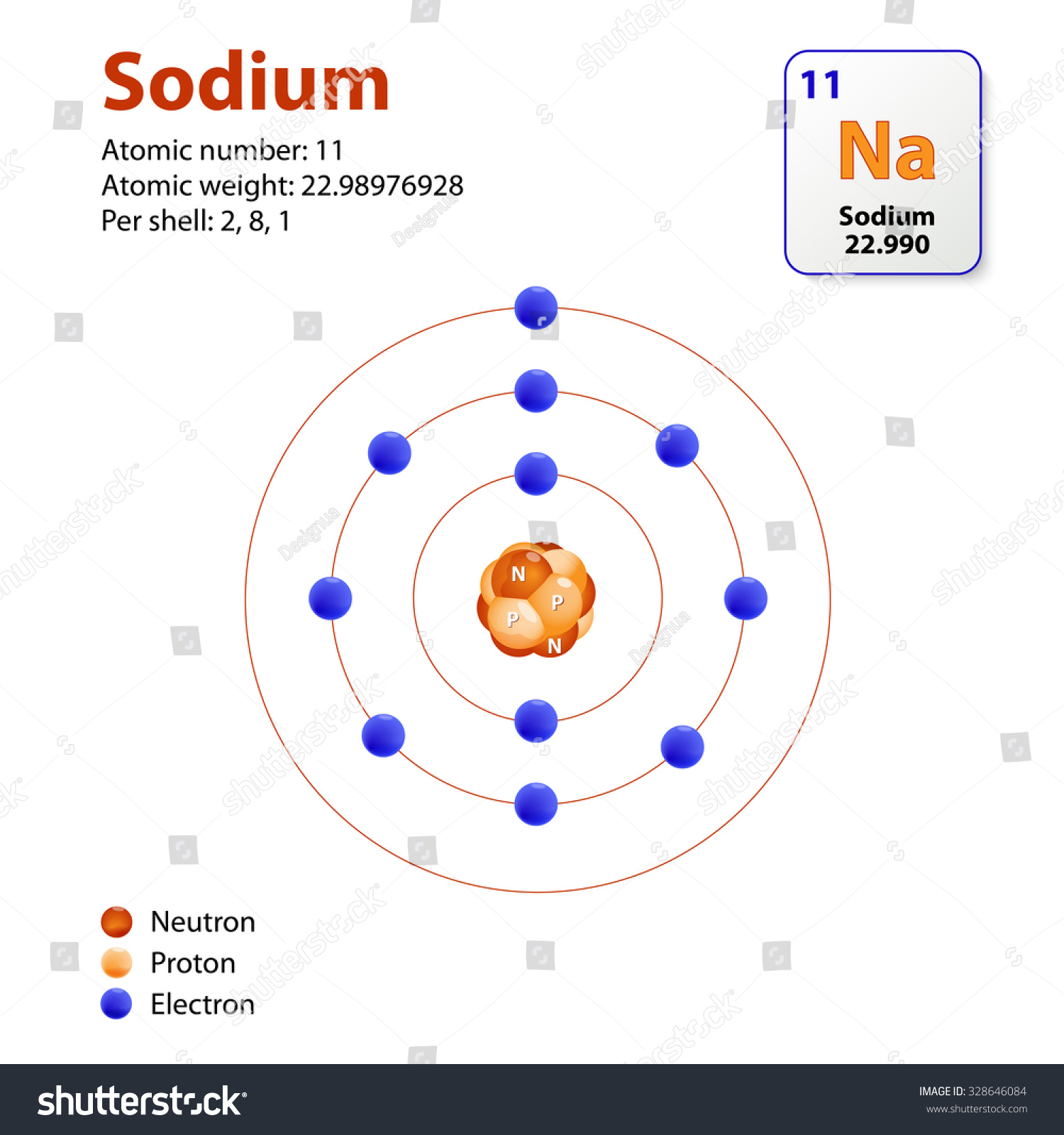
Sodium (Na) is the first element in the 3rd row (Period 3) in the periodic table. This means that the first shell and second shells of Na atom are filled to the maximum number of electrons. The first shell (1s) is filled with 2 electrons. The second shell (2s and 2p) has a total of 8 electrons. And, the third (last) shell has 1 electron.

Electron Configuration for Sodium (Na, and Na+ ion)
We can show the electron arrangement as (2, 8, 2) representing the electrons in the n = 1 n = 1, n = 2 n = 2, and n = 3 n = 3 levels, respectively. Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3.
Sodium atom polizworth
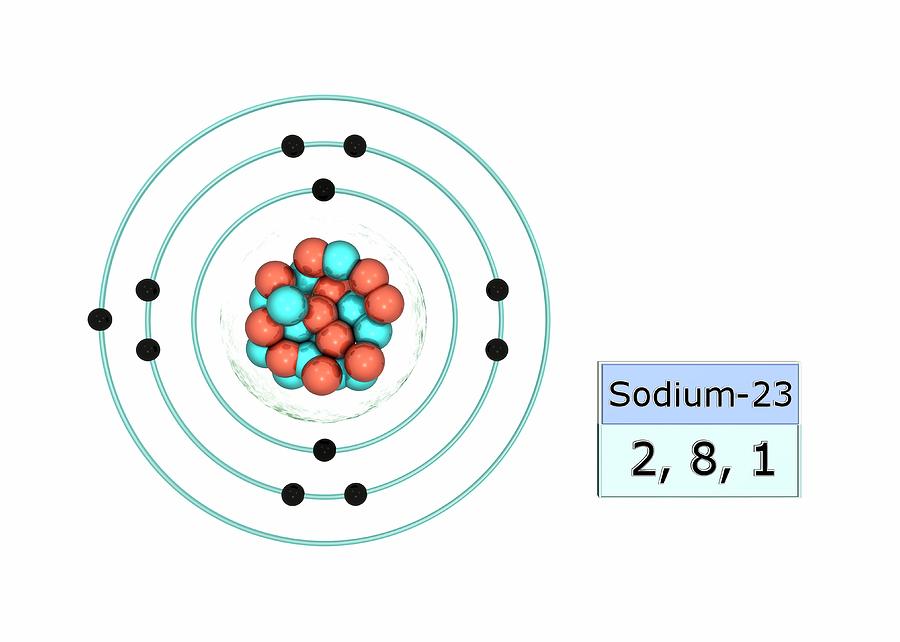
Stable Isotopes Electrons and Electron Configuration The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Sodium is 11.
Electron arrangements
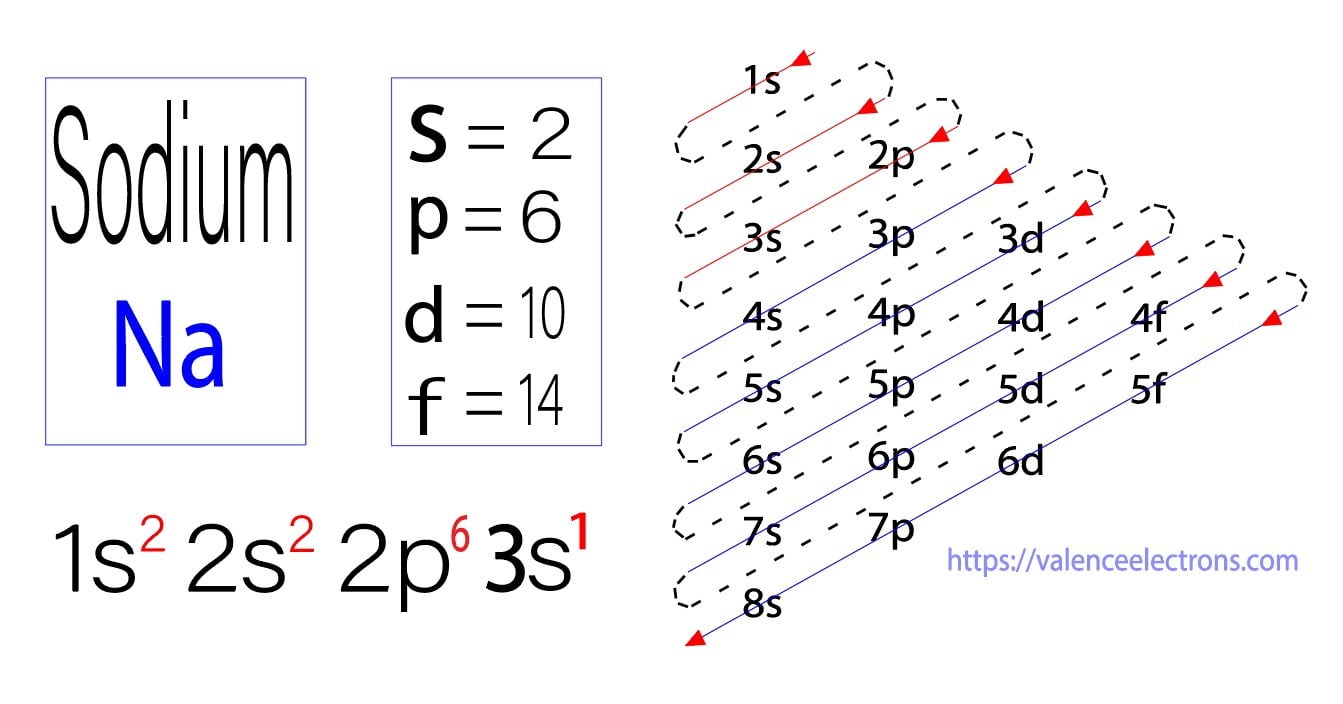
The electron configuration of a neutral sodium atom is 1s^2 2s^2 2p^6 3s^1. In this configuration we note that there is only one electron in the 3rd energy level. Atoms prefer to gain the stability of octet, by having eight electrons in the outer shell, the electrons of the s and p orbitals.
Sodium Bohr Diagram
March 23, 2023 by Jay. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
Electron Configuration for Sodium (Na, and Na+ ion)
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).. An orbital diagram is the more visual way.
Sodium Electron Configuration Electron Configuration Sodium What is
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid-liquid phase change occurs.. Sodium is essential to all living things, and humans have known this since prehistoric times. Our bodies contain about 100 grams, but we are constantly losing sodium in.

Introduction to Atoms
. They begin to occupy the next shell only when this shell becomes full. For elements with atomic number 1 to 20: Predicting an electron arrangement The electron arrangement of an atom can be.
Sodium Electron Configuration Photograph by Photo
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,

Sodium electronic configuration How to Write Sodium electronic
If we look at the arrangement of electrons in a sodium atom, we will find that two electrons fill the first shell, eight electrons fill the second shell, and there's one electron left to go in the third shell. But the question remains.

FileElectron shell 011 sodium.png Wikimedia Commons
Because lithium's final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s 2 2s 1. The shell diagram for a lithium atom is shown below. The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1 s , while the outermost shell ( 2 s ) has 1 electron.
This Diagram Shows The Electron Shell Configuration For The Sodium Atom
The arrangement of electrons in sodium in specific rules in different orbits and orbitals is called the electron configuration of sodium. The electron configuration of sodium is [ Ne] 3s 1 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)
Sodium Electron Configuration (Na) with Orbital Diagram
The electron arrangement of an element is related to its position on the periodic table. The electron arrangement of sodium (2.8.1) shows that sodium, Na: is in period 3 is in group 1.

Atom Sodium Model Stock Illustration Download Image Now iStock
The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. Orbital Energies and Atomic Structure.. Sodium cation loses one electron, so Na +: 1s 2 2s 2 2p 6 3s 1 = Na +: 1s 2 2s 2 2p 6. P: 1s 2 2s 2 2p 6 3s 2 3p 3.